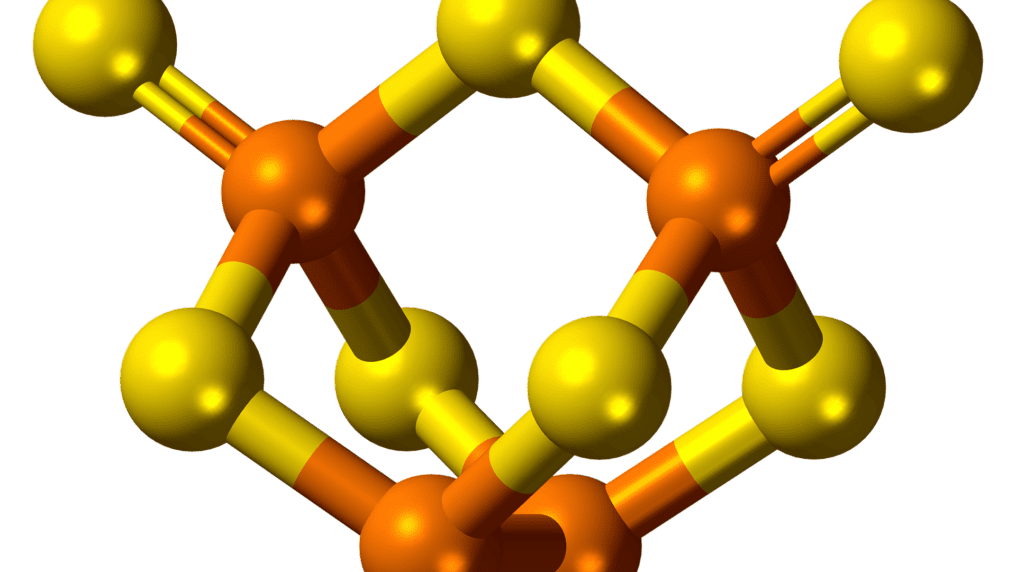
Phosphorus is the element corresponding to the atomic number 15 in the modern periodic table. It is denoted by the symbol ‘P’. Phosphorus cannot be found as a free element on Earth due to its highly reactive nature. It exists in the form of white phosphorus and red phosphorus. There are 15 electrons in the atomic structure of phosphorus. Some more chemical properties and applications of phosphorus are listed below.
Some Chemical Properties and Applications of Phosphorus:
Phosphorus: History and Invention:
A German scientist, Hennig Brand, discovered the chemical element, phosphorus in 1669. As compared to other elements, phosphorus is a rather interesting element. The element occurs in 3 completely different forms- naturally, it is so reactive, that pure phosphorous will immediately catch fire if exposed to air. If you view phosphorus in its natural form at night, it glows in the dark, which is quite fascinating to look at. However, in modern times, phosphorus is used solely in its liquid form, by converting it into an acid called Phosphoric Acid (H3PO4). This acid is used to manufacture fertilizers and has found other usages in the agricultural and metallurgical industries.
Generally, minerals that contain phosphorus are generally phosphate rocks in which phosphorus is oxidized to PO43-. The group of elements including Phosphorus, Nitrogen, Arsenic, Antimony, and Bismuth makes up the Pnictogens.
Where is Phosphorus Found on Earth?
As compared to other naturally found elements in the Earth’s crust, Phosphorus amounts to 012%, which means that it is the 11th most commonly found element on Earth. Most of the Phosphorus is found naturally in North America, with Florida and North Carolina accounting for most of the world’s Phosphorus production. Some other countries where phosphorus is found are Israel, Jordan, Russia, Tunisia, Morocco, and China.
About 86% of phosphate rock comes from North Carolina and Florida. Smaller amounts are also mined in Idaho and Utah. Other major producers of phosphate rock are Morocco, China, Russia, Tunisia, Jordan, and Israel.
Phosphorus: Uses and Requirements:
Phosphorus is an essential part of life and phosphate-containing compounds are a part of DNA and RNA. A good chunk of the phosphorus compounds mined from the planet Earth find applications as fertilizers.
Phosphates are required to renew soils which have been stripped of it by plants. Phosphorus is a p block element having the electronic configuration of [Ne]3s23p3. Its standard atomic weight is approximately 30.9737. At standard conditions for temperature and pressure, phosphorus is found in the solid state.
Phosphorus: Chemical and Physical Properties:
The melting point of phosphorus is 317.5K and its boiling point is 553.7K. It can exhibit oxidation states ranging from -3 to +5. The Van der Waals radius corresponding to a phosphorus atom is 180 pm. It has an electronegativity of 2.19 on the Pauling scale.
Phosphorus is also a primary culprit for eutrophication when it is present in excess in any ecosystem since it promotes algae blooms. Deficiency of phosphorus in human beings is characterized by the condition called hypophosphatemia and can cause neurological dysfunction along with disruption of blood cells and muscle cells.
To learn more about other important elements in the periodic table, such as carbon, subscribe to the BYJU’S youtube channel and enable notifications.
Read Also:
- Discover Croatia with Nautal
- Learn Arabic in a Snap with the Best Arabic Language Apps
- Info Savvy: Perfect Educational and Economical Gizmos for Kids
- STEM In Real Life: 5 Awesome STEM Projects for High Schoolers
How Personal Development Books For College Students Can Save You Time, Stress, and Money